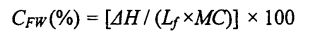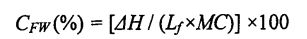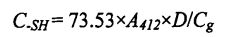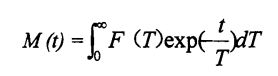Improving the processing properties of frozen dough has certain practical significance for realizing large-scale production of high-quality convenient steamed bread. In this study, a new type of hydrophilic colloid (hydroxypropyl methylcellulose, Yang, MC) was applied to frozen dough. The effects of 0.5%, 1%, 2%) on the processing properties of frozen dough and the quality of steamed bread were evaluated to evaluate the improvement effect of HPMC. Faatosinaga i luga o le fausaga ma meatotino o vaega (saito goto, saito starch ma le fefete).
O le mea e gaosia ai le gaosiga o le meafaitino o le meafaitino o le meafaitino o le mea e mafua ai le taua o le lelei o le vaega o le vaituloto o mea. Through experiments, it was found that, compared with the control group, the addition of HPMC could better maintain the fermentation activity of yeast and reduce the increase rate of extracellular reduced glutathione content after 60 days of freezing, and within a certain range, The protective effect of HPMC was positively correlated with its addition amount.
Lisi o mea
1.1.3 Frozen Pagh folasaga folamo .........................................................................................................................
1.1.6 Faʻaaogaina o Hydrocolloads i Frozen Paʻu Lelei Faʻalelei ..................... .5
1.1.7 Hydroxypropyl methyl cellulose (Hydroxypropyl methyl cellulose, I-IPMC) ………. 5
112 Faamoemoega ma le Taua o le Suʻesuʻega ................................................................................ 6
2.3 Experimental results and discussion…………………………………………………………………… . 11
2.3.3 O le aʻafiaga o le HPMC faʻaopopo i luga o le telē o le paluga .............................. 12
3.2.4 Experimental methods ....................................................................................................... 25
3.3.2 The effect of adding amount of HPMC and freezing storage time on the freezable moisture content (CFW) and thermal stability……………………………………………………………………. 30
3.3.3 Effects of HPMC addition amount and freezing storage time on free sulfhydryl content (C vessel) …………………………………………………………………………………………………………. . 34
3.4 Mataupu Aotelega ................................................................................................................................................. 43
4.1 Introduction .............................................................................................................................. . 44
4.2 Faʻamatalaga o Faʻamatalaga ma metotia ......................................................................................................... 45
4.3 Analysis and discussion ........................................................................................................... 48
4.3.1 Content of basic components of wheat starch ……………………………………………………. 48
4.3.3 Effects of HPMC addition and freezing storage time on the shear viscosity of starch paste………………………………………………………………………………………………………………………………………. 52
4.3.6 Effects of I-IPMC addition amount and frozen storage time on the thermodynamic properties of starch ………………………………………………………………………………………………………. . 57
4.4 Mataupu Aotelega .................................................................................................................................................. 6 1
Chapter 5 Effects of HPMC addition on yeast survival rate and fermentation activity under frozen storage conditions………………………………………………………………………………………………. . 62
5.1Introduction .................................................................................................................................... 62
5.2 Materials and methods ............................................................................................................ 62
5.2.2 Experimental methods . . . . . …………………………………………………………………………. 63
5.3 Results and Discussion ............................................................................................................... 64
5.3.3 The effect of adding amount of HPMC and freezing time on the content of glutathione in dough……………………………………………………………………………………………………………66. "
5.4 Chapter Summary ........................................................................................................................ 67
6.2 Outlook .......................................................................................................................................... 68
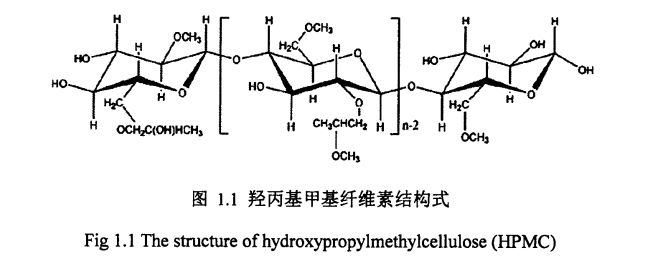
Ata 1.1 O le fausaga faʻavae o Hydroxypropyl methylcelsulose ............................ . 6
Figure 2.4 The effect of HPMC addition and freezing time on the elasticity of steamed bread………………………………………………………………………………………………………………………………. . 20
Figure 3.1 The effect of HPMC addition and freezing time on the rheological properties of wet gluten…………………………………………………………………………………………………………………………. 30
Figure 3.2 Effects of HPMC addition and freezing time on the thermodynamic properties of wheat gluten………………………………………………………………………………………………………………. . 34
Figure 3.3 Effects of HPMC addition and freezing time on free sulfhydryl content of wheat gluten……………………………………………………………………………………………………………………………... . 35
Figure 3.7 The effect of HPMC addition and freezing time on the microscopic gluten network structure…………………………………………………………………………………………………………... . 43
Figure 4.1 Starch gelatinization characteristic curve ............................................................... 51
Figure 4.2 Fluid thixotropy of starch paste ................................................................................. 52
Figure 4.3 Effects of adding amount of MC and freezing time on the viscoelasticity of starch paste……………………………………………………………………………………………………………………... . 57
Figure 4.5 Effects of HPMC addition and freezing storage time on the thermodynamic properties of starch…………………………………………………………………………………………………………. . 59
Figure 5.2 The effect of HPMC addition and freezing time on the yeast survival rate…………………………………………………………………………………………………………………………………... . 67
Figure 5.3 Microscopic observation of yeast (microscopic examination) …………………………………………………………………………………………………………………………. 68
Table 2.1 The basic ingredient content of wheat flour…………………………………………………. 11
Lisi 2.3 Aafiaga ole I-IPMC faʻaopopo i luga o le paluga le paluga ....................................... .14
Table 3.2 Effects of I-IPMC addition amount and freezing storage time on the phase transition enthalpy (Yi IV) and freezer water content (e chat) of wet gluten………………………. 31
Table 3.3 Effects of HPMC addition amount and freezing storage time on the peak temperature (product) of thermal denaturation of wheat gluten…………………………………………. 33
Table 3.6 Effects of I-IPMC addition and freezing storage time on the surface hydrophobicity of wheat gluten……………………………………………………………………………………………. 41
Table 4.3 Effects of I-IPMC addition and freezing time on the shear viscosity of wheat starch paste…………………………………………………………………………………………………………………………. 55
1.1reserch tulaga i le fale ma fafo
1.1.1Faʻaiga i le falaoa o le falaoa
Steamed bread refers to the food made from the dough after proofing and steaming. As a traditional Chinese pasta food, steamed bread has a long history and is known as "Oriental Bread". Because its finished product is hemispherical or elongated in shape, soft in taste, delicious in taste and rich in nutrients [l], it has been widely popular among the public for a long time. O meaʻai faʻatutu a tatou atunuʻu, aemaise lava ile itu o loʻo i le itu o loʻo iai i le itu. The consumption accounts for about 2/3 of the dietary structure of products in the north, and about 46% of the dietary structure of flour products in the country [21].
1) Atinae o fou uiga fou uamea kunu. Through the innovation of steamed bread raw materials and the addition of functional active substances, new varieties of steamed breads have been developed, which have both nutrition and function. Established the evaluation standard for the quality of miscellaneous grain steamed bread by principal component analysis; Fu et a1. (2015) added lemon pomace containing dietary fiber and polyphenols to steamed bread, and evaluated the antioxidant activity of steamed bread; Hao & Beta (2012) studied barley bran and flaxseed (rich in bioactive substances) The production process of steamed bread [5]; Shiau et a1. (2015) evaluated the effect of adding pineapple pulp fiber on dough rheological properties and steamed bread quality [6].
2)Research on the processing and compounding of special flour for steamed bread. The effect of flour properties on the quality of dough and steamed buns and the research on new special flour for steamed buns, and based on this, an evaluation model of flour processing suitability was established [7]; Faʻataʻitaʻiga, o aʻafiaga o isi feʻela tele fauina o le lelei o auala falaoamata ma faʻapipiʻi kuka [7] 8; The effect of the compounding of several waxy wheat flours on the quality of steamed bread [9J et al.; Zhu, Huang, &Khan (2001) evaluated the effect of wheat protein on the quality of dough and northern steamed bread, and considered that gliadin/ Glutenin was significantly negatively correlated with dough properties and steamed bread quality [lo]; Zhang, et a1. (2007) analyzed the correlation between gluten protein content, protein type, dough properties and steamed bread quality, and concluded that the content of high molecular weight glutenin subunit (1ligh.molecular-weight, HMW) and total protein content are all related to the quality of northern steamed bread. have a significant impact [11].
3)Research on dough preparation and steamed bread making technology. Suʻesuʻega i le faʻatosinaga o fola o falaoa uamea foaʻiina i lona tulaga lelei ma faʻagaeetia lelei; Liu Changhong et al. (2009) showed that in the process of dough conditioning, process parameters such as water addition, dough mixing time, and dough pH value have an impact on the whiteness value of steamed bread. It has a significant impact on sensory evaluation. If the process conditions are not suitable, it will cause the product to turn blue, dark or yellow. The research results show that during the dough preparation process, the amount of water added reaches 45%, and the dough mixing time is 5 minutes, ~ When the pH value of the dough was 6.5 for 10 min, the whiteness value and sensory evaluation of the steamed buns measured by the whiteness meter were the best. Pe a fai le paluga 15-20 taimi i le taimi e tasi, o le paluga o le lamolemole, lamolemole, fatafata ma le lūlaupu luga; A faʻaitiitia le fuata mai le 3: 1, o le tata o le tino, ma o le paʻepaʻe o le areto na faʻaputuputu [L e; Li, et a1. (2015) Faʻaaogaina le gaosiga o paluga faʻapipiʻi ma lona apalai i le fasi falaoa e gaosi [13].
5)Preservation and anti-aging of steamed bread and related mechanisms. Pan Lijun et al. (2010) optimized the composite modifier with good anti-aging effect through experimental design [l do not; Wang, et a1. (2015) studied the effects of gluten protein polymerization degree, moisture, and starch recrystallization on the increase of steamed bread hardness by analyzing the physical and chemical properties of steamed bread. O iʻuga na faʻaalia ai o le vai leiloa ma starch reterystallization o mafuaʻaga autu lava o le maʻi o le la falaoa [20].
6)Research on the application of new fermented bacteria and sourdough. Jiang, et a1. (2010) Application of Chaetomium sp. ua uma ona maua ai le xylanase (ma le totoa) i le areto uamea [2l '; Gerez, et a1. (2012) used two kinds of lactic acid bacteria in fermented flour products and evaluated their quality [221; Wu, et al. (2012) studied the influence of sourdough fermented by four kinds of lactic acid bacteria (Lactobacillus plantarum, Lactobacillus, sanfranciscemis , Lactobacillus brevis and Lactobacillus delbrueckii subsp bulgaricus) on the quality (specific volume, texture, fermentation flavor, etc.) of northern steamed bread [23]; and Gerez, et a1. (2012) used the fermentation characteristics of two kinds of lactic acid bacteria to accelerate the hydrolysis of gliadin to reduce the allergenicity of flour products [24] and other aspects.
Frozen dough is a new technology for the processing and production of flour products developed in the 1950s. E faʻapea lava o loʻo faʻasino i le faʻaaogaina o le saito o le falaoamata e pei o mea autu poʻo le vai poʻo le suka e pei o mea taua o ausilali. Baked, packed or unpacked, quick-freezing and other processes make the product reach a frozen state, and in. For products frozen at 18"C, the final product needs to be thawed, proofed, cooked, etc. [251].
a)How to restrain the quality deterioration of frozen dough with the extension of frozen storage time, especially how to control the influence of the formation and growth of ice crystals on the structure and properties of the three main components of dough (starch, gluten and yeast), is still an issue. Hotspots ma Mataupu taua i lenei suʻesuʻega fanua;
1.1.5reserch tulaga o le paluga faasee
In view of the above problems and challenges of frozen dough, the long-term innovative research on the application of frozen dough technology, the quality control and improvement of frozen dough products, and the related mechanism of changes in the structure and properties of material components in the frozen dough system and quality deterioration Such research is a hot issue in the field of frozen dough research in recent years. A faʻapitoa, o le vaega autu o aiga ma fafo atu i tausaga talu ai nei e tele vaʻaia manatu ia:
i.Study the changes in the structure and properties of frozen dough with the extension of freezing storage time, in order to explore the reasons for the deterioration of product quality, especially the effect of ice crystallization on biological macromolecules (protein, starch, etc.), for example, ice crystallization. Faʻavae ma le tuputupu aʻe ma lona fesoʻotaʻiga ma le setete ma le setete ma le atunuʻu ma tufatufa atu; suiga i le saito sclut defertini fausaga, tuufaatasiga ma meatotino [31]; suiga ile scarction fausaga ma meatotino; Suiga i le paluga palu ma faʻamatalaga faʻatatau i mea totino, ma isi. 361.
Studies have shown that the main reasons for the deterioration of the processing properties of frozen dough include: 1) During the freezing process, the survival of yeast and its fermentation activity are significantly reduced; 2) Le faʻatulagaina o fesoʻotaʻiga ma le atoatoa o le paluga ua faatamaia, e mafua ai le le umiaina o le ea gafatia le malosi o le paluga. ma le malosi o le malosi ua faʻaititia tele.
Ii. Optimization of frozen dough production process, frozen storage conditions and formula. During the production of frozen dough, temperature control, proofing conditions, pre-freezing treatment, freezing rate, freezing conditions, moisture content, gluten protein content, and thawing methods will all affect the processing properties of frozen dough [37]. In general, higher freezing rates produce ice crystals that are smaller in size and more uniformly distributed, while lower freezing rates produce larger ice crystals that are not uniformly distributed. In addition, a lower freezing temperature even below the glass transition temperature (CTA) can effectively maintain its quality, but the cost is higher, and the actual production and cold chain transportation temperatures are usually small. In addition, the fluctuation of the freezing temperature will cause recrystallization, which will affect the quality of the dough.
III. Using additives to improve the product quality of frozen dough. In order to improve the product quality of frozen dough, many researchers have made explorations from different perspectives, for example, improving the low temperature tolerance of material components in frozen dough, using additives to maintain the stability of the dough network structure [45.56], etc. Among them, the use of additives is an effective and widely used method. Mainly include, i) enzyme preparations, such as, transglutaminase, O [. Amylase; ii) EMAU ITusififis, pei o Monogalyceridede Storarate, Aso, SSL, CSL, aso malolo, ma isi.; iii) antioxidants, ascorbic acid, etc.; iv) polysaccharide hydrocolloids, such as guar gum, yellow Originalgum, gum Arabic, konjac gum, sodium alginate, etc.; v) other functional substances, such as Xu, et a1. (2009) added Ice-structuring Proteins to wet gluten mass under freezing conditions, and studied its protective effect and mechanism on the structure and function of gluten protein [y71.
The chemical nature of hydrocolloid is a polysaccharide, which is composed of monosaccharides (glucose, rhamnose, arabinose, mannose, etc.) through 0 [. 1-4. Glycosidic bond po o / ma a. 1--"6. Glycosidic bond or B. 1-4. Glycosidic bond and 0 [.1-3. The high molecular organic compound formed by the condensation of glycosidic bond has a rich variety and can be roughly divided into: ① Cellulose derivatives , such as methyl cellulose (MC), carboxymethyl cellulose (CMC); ② plant polysaccharides, such as konjac gum, guar gum, gum Arabic ; ③ seaweed polysaccharides, such as seaweed gum, carrageenan; ④ microbial polysaccharides, such as Xanthan gum .Polysaccharide has strong hydrophilicity because it contains a large number of hydroxyl groups that are easy to form hydrogen bonds with water, and has the functions of controlling the migration, state and distribution of water in the food system. Therefore, the addition of hydrophilic colloids gives food Many functions, properties, and qualities of hydrocolloids are closely related to the interaction between polysaccharides and water and other macromolecular substances. At the same time, due to the multiple functions of thickening, stabilizing, and water retention, hydrocolloids are widely used to include in the food processing of flour products. Wang Xin et al. (2007) studied the effect of adding seaweed polysaccharides and gelatin on the glass transition temperature of dough [631. Wang Yusheng et al. (2013) believed that compound addition of a variety of hydrophilic colloids can significantly change the flow of dough. Change the properties, improve the tensile strength of the dough, enhance the elasticity of the dough, but reduce the extensibility of the dough [delete.
1.1.7hydroxyproxpyl methyl cellulose (hydroxyproxpyl methyl cellulose, I-IPMC)
1.2resech Faamoemoega ma taua
Generally speaking, the material composition of dough used for making fermented flour products mainly includes biological macromolecular substances (starch, protein), inorganic water, and yeast of organisms, and is formed after hydration, cross-linking and interaction. O se nofoaga mautu ma le faigata o mea ma se fausaga faʻapitoa ua atiaʻe. Numerous studies have shown that the properties of the dough have a significant impact on the quality of the final product. Therefore, by optimizing the compounding to meet the specific product and it is a research direction to improve the dough formulation and technology of the quality of the product or food for use; on the other hand, improving or improving the properties of dough processing and preservation to ensure or improve the quality of the product is also an important research issue.
2.2 faʻataʻitaʻiga mea ma metotia
Zhongyu Wheat Flour Binzhou Zhongyu Food Co., Ltd.; Angel Active Dry Yeast Angel Yeast Co., Ltd.; HPMC (methyl suia tikeri o le 28% .30%, Hydroxypropyver Suiga o le 7% .12%) Vailaau Company Company O vailaʻau uma o loʻo faʻaaogaina sa faʻaaogaina i lenei faʻataʻitaʻiga o le vasega vasega;
2.2.2 Gaoioiga Gaoioiga ma Meafaigaluega
TA-LTT PRIMER O LE AIGA O LE AIGA
C21. KT2134 Induction Cooker
Powder mita. U
Faalauteleina. U
Mauaina R3 Rourtional Rheometer
Fd. 1b. 50 VIFUUM FREEZ MULIMULI
Tagata gaosi
2.2.3.1 Faʻamoemoeina o vaega faʻavae o falaoamata
2.2.3.2 Fuafuaina o le Floray meatotino o paluga
2.2.3.3.3 Faʻamalologa o le tenisi meatotino o paluga
Fuafuaina o le tenisi meatotino o paluga tusa ma GB / T 14615.2006 [831.
A sample (about 2 g) of the central part of the partially melted dough was cut and placed on the bottom plate of the rheometer (Discovery R3). First, the sample was subjected to dynamic strain scanning. The specific experimental parameters were set as follows: A parallel plate with a diameter of 40 mm was used, the gap was set to 1000 mln, the temperature was 25 °C, and the scanning range was 0.01%. 100%, o le faʻataʻitaʻiga o le taimi o le taimi o le 10 min, ma ua uma ona faʻatulaga i le 1hz. O le laina o le laina o le laina o le pule o loʻo iai (lvr) o faʻataʻitaʻiga sa faʻataʻitaʻi na fuafuaina e le syin scaning. Then, the sample was subjected to a dynamic frequency sweep, and the specific parameters were set as follows: the strain value was 0.5% (in the LVR range), the resting time, the fixture used, the spacing, and the temperature were all consistent with the strain sweep parameter settings. Five data points (plots) were recorded in the rheology curve for each 10-fold increase in frequency (linear mode). After each clamp depression, the excess sample was gently scraped with a blade, and a layer of paraffin oil was applied to the edge of the sample to prevent water loss during the experiment. Each sample was repeated three times.
2.2.3.6 Anotusi o le suavai suavai (mea o loʻo i totonu o le vai, cf i totonu)
Faatasi ma i latou, 厶 o loʻo faatusa i le vevela o le susā o le susu, ma lona taua o le 334 ma le Dan; MC (total Moisture Content) represents the total moisture content in the dough (measured according to GB 50093.2010t78]). Each sample was repeated three times.
After the corresponding freezing time, the frozen dough was taken out, first equilibrated in a 4°C refrigerator for 4 h, and then placed at room temperature until the frozen dough was completely thawed. Divide the dough into about 70 grams per portion, knead it into shape, and then put it into a constant temperature and humidity box, and proof it for 60 minutes at 30°C and a relative humidity of 85%. Ina ua uma ona faʻamaonia, Steam mo 20 min, ona malu lea mo le 1 h i le potu vevela e iloilo ai le lelei o le falaoa uʻamea.
Steed falaoa faʻapitoa (cm3 / g) = falaoa falaoa (cm3) / faʻapipiʻi falaoa tele (g)
Refer to the method of Sim, Noor Aziah, Cheng (2011) [88] with minor modifications. A 20x 20 x 20 mn'13 core sample of the steamed bread was cut from the central area of the steamed bread, and the TPA (Texture Profile Analysis) of the steamed bread was measured by a physical property tester. Specific parameters: the probe is P/100, the pre-measurement rate is 1 mm/s, the mid-measurement rate is 1 mm/s, the post-measurement rate is 1 mm/s, the compression deformation variable is 50%, and the time interval between two compressions is 30 S, the trigger force is 5 g. O faʻataʻitaʻiga taʻitasi na toe faia 6 taimi.
2.3 faʻataʻitaʻiga faʻaiuga ma talanoaga
2.3.2 O le aʻafiaga o I-IPMC faʻaopopo i luga o mea faifaatoaga o le paluga
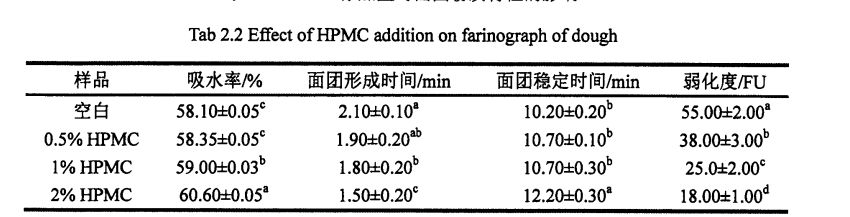
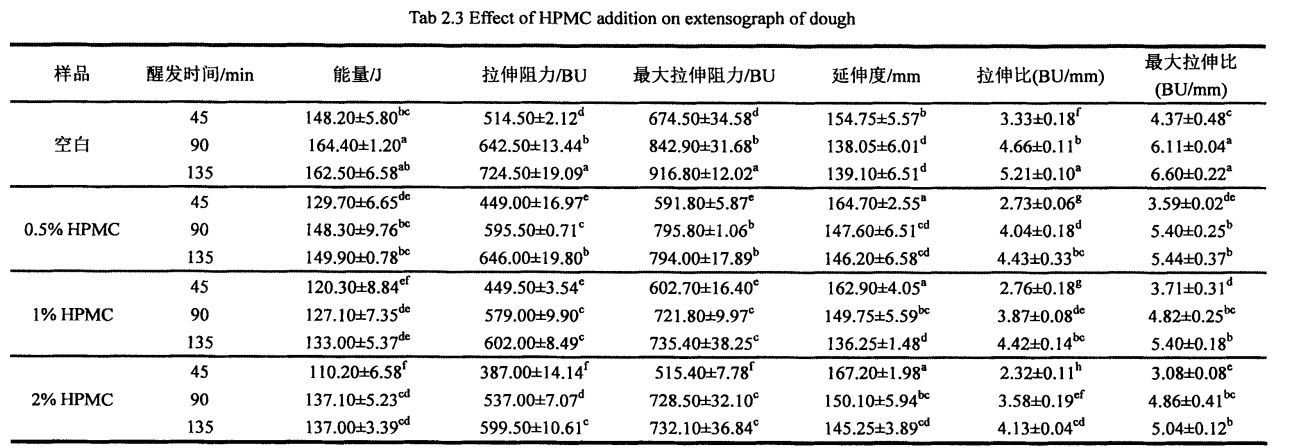
As shown in Table 2.2, with the increase of HPMC addition, the water absorption of dough increased significantly, from 58.10% (without adding HPMC dough) to 60.60% (adding 2% HPMC dough). I le faʻaopopoga, o le faʻaopopoga o le HPMC na faʻaleleia le paluga mautu taimi mai le 10.2 min (avanoa) i le 12.2 Min (faʻaopoopo 2% HPMC). However, with the increase of HPMC addition, both the dough forming time and the dough weakening degree decreased significantly, from the blank dough forming time of 2.10 min and the weakening degree of 55.0 FU, respectively, to the addition of 2% HPMC, the dough forming time was 1. .50 min and weakening degree of 18.0 FU, decreased by 28.57% and 67.27%, respectively.
Peitaʻi, o le faʻaopopoina o le HPMC e mafai ona ofi atu i luga ma le tele o mea i luga ma sui o meatotino faʻatele o le paluga. With the increase of HPMC addition, the tensile resistance, maximum tensile resistance and energy value of the dough all decreased correspondingly, while the elongation increased. Specifically, when the proofing time was 45 min, with the increase of HPMC addition, the dough energy value decreased significantly, from 148.20-J: 5.80 J (blank) to 129.70-J respectively: 6.65 J (add 0.5% HPMC), 120.30 ± 8.84 J (add 1% HPMC), and 110.20-a: 6.58
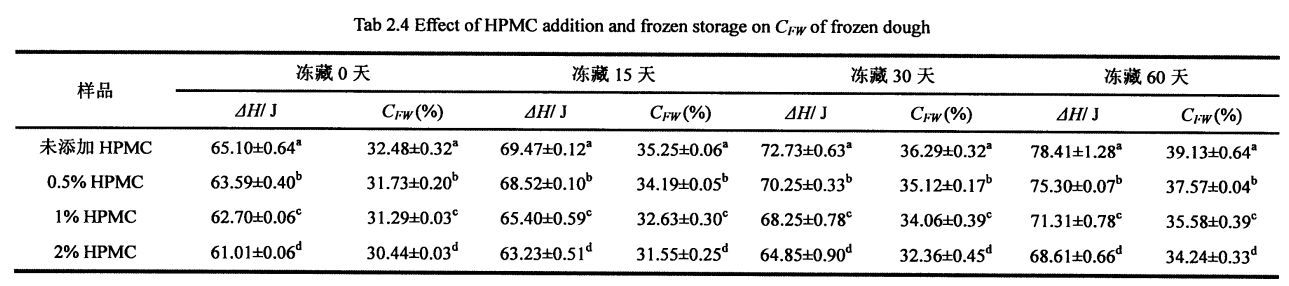
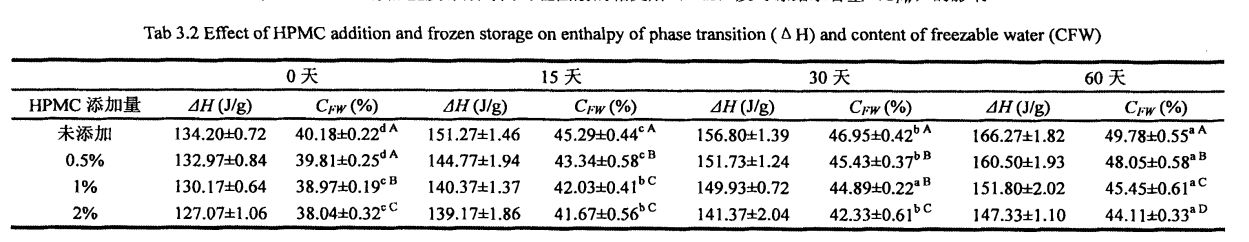
Not all the moisture in the dough can form ice crystals at a certain low temperature, which is related to the state of the moisture (free-flowing, restricted, combined with other substances, etc.) and its environment. Freezable water is the water in the dough that can undergo phase transformation to form ice crystals at low temperatures. The amount of freezable water directly affects the number, size and distribution of ice crystal formation. In addition, the freezable water content is also affected by environmental changes, such as the extension of freezing storage time, the fluctuation of freezing storage temperature, and the change of material system structure and properties. For the frozen dough without added HPMC, with the prolongation of freezing storage time, Q silicon increased significantly, from 32.48±0.32% (frozen storage for 0 days) to 39.13±0.64% (frozen storage for 0 days). O Tibetan mo 60 aso), o le siʻitaga o le 20.47%. However, after 60 days of frozen storage, with the increase of HPMC addition, the increase rate of CFW decreased, followed by 18.41%, 13.71%, and 12.48% (Table 2.4). At the same time, the o∥ of the unfrozen dough decreased correspondingly with the increase of the amount of HPMC added, from 32.48a-0.32% (without adding HPMC) to 31.73±0.20% in turn. (adding0.5% HPMC), 3 1.29+0.03% (adding 1% HPMC) and 30.44±0.03% (adding 2% HPMC) Water holding capacity, inhibits the free flow of water and reduces the amount of water that can be frozen. In the process of freezing storage, along with recrystallization, the dough structure is destroyed, so that part of the non-freezable water is converted into freezable water, thus increasing the content of freezable water. However, HPMC can effectively inhibit the formation and growth of ice crystals and protect the stability of the dough structure, thus effectively inhibiting the increase of the freezable water content. This is consistent with the change law of the freezable water content in the frozen wet gluten dough, but because the dough contains more starch, the CFW value is smaller than the G∥ value determined by the wet gluten dough (Table 3.2).
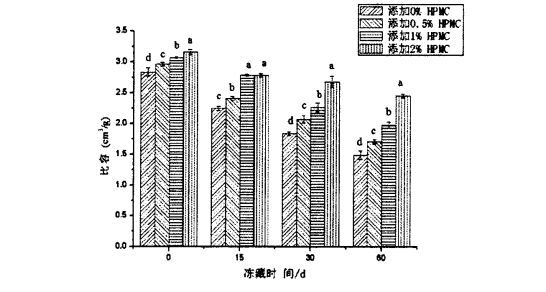
The specific volume of steamed bread can better reflect the appearance and sensory quality of steamed bread. The larger the specific volume of the steamed bread, the larger the volume of the steamed bread of the same quality, and the specific volume has a certain influence on the appearance, color, texture, and sensory evaluation of the food. Ile taimi e masani ai ona tautala, uamea ma lapisi ma lapoa faʻapitoa volume e sili atu ona lauiloa foi i tagata faʻatau atu i se isi tulaga.
Sti 2.2 Aafiaga o HPMC Seam ma Frozen Teuina i luga o vaega faapitoa o le Saina Steed Areto
The specific volume of steamed bread can better reflect the appearance and sensory quality of steamed bread. The larger the specific volume of the steamed bread, the larger the volume of the steamed bread of the same quality, and the specific volume has a certain influence on the appearance, color, texture, and sensory evaluation of the food. Ile taimi e masani ai ona tautala, uamea ma lapisi ma lapoa faʻapitoa volume e sili atu ona lauiloa foi i tagata faʻatau atu i se isi tulaga.
However, the specific volume of the steamed bread made from frozen dough decreased with the extension of the frozen storage time. Among them, the specific volume of the steamed bread made from the frozen dough without adding HPMC was 2.835±0.064 cm3/g (frozen storage). 0 days) down to 1.495±0.070 cm3/g (frozen storage for 60 days); A o le vaega faʻapitoa o falaoa na lafo mai ai le paluga faʻi ua faʻaopopoina ma le 2% hpmc pa'ū mai le 3.160 ± 0.04 ± 0.04 _ 0.041 451±0.033 cm3/g, therefore, the specific volume of the steamed bread made from the frozen dough added with HPMC decreased with the increase of the added amount. Since the specific volume of steamed bread is not only affected by the yeast fermentation activity (fermentation gas production), the moderate gas holding capacity of the dough network structure also has an important impact on the specific volume of the final product [96'9 cited. The measurement results of the above rheological properties show that the integrity and structural strength of the dough network structure are destroyed during the freezing storage process, and the degree of damage is intensified with the extension of the freezing storage time. I le taimi o le faʻagasologa, o le kesi o loʻo taofia ai le malosi e le lelei, lea e oʻo ai i le faʻaititia i le faʻaititia o le vaega faʻapitoa o le falaoa uamea. However, the addition of HPMC can more effectively protect the integrity of the dough network structure, so that the air-holding properties of the dough are better maintained, therefore, in O. During the 60-day frozen storage period, with the increase of HPMC addition, the specific volume of the corresponding steamed bread decreased gradually.
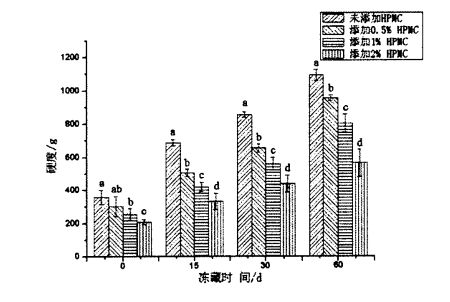
TPA (Textural Profile Analyses) physical property test can comprehensively reflect the mechanical properties and quality of pasta food, including hardness, elasticity, cohesion, chewiness and resilience. Figure 2.3 shows the effect of HPMC addition and freezing time on the hardness of steamed bread. The results show that for fresh dough without freezing treatment, with the increase of HPMC addition, the hardness of steamed bread significantly increases. decreased from 355.55±24.65g (blank sample) to 310.48±20.09 g (add O.5% HPMC), 258.06±20.99 g (add 1% t-IPMC) and 215.29 + 13.37 g (2% HPMC added). E ono fesoʻotaʻi lea ma le siʻitia o le tele o vaega o falaoa uʻamea. In addition, as can be seen from Figure 2.4, as the amount of HPMC added increases, the springiness of steamed bread made from fresh dough increases significantly, from 0.968 ± 0.006 (blank) to 1, respectively. .020 ± 0.004 (Faʻaopopo 0.5% HPMC), 1.073 ± 0.006 1% HPMC). O suiga o le faigata ma le manaia o le areto ua tuʻu mai o le faʻaopopoga o le HPMC e mafai ona faʻaleleia le lelei o le falaoa uamea. This is consistent with the research results of Rosell, Rojas, Benedito de Barber (2001) [95] and Barcenas, Rosell (2005) [worms], that is, HPMC can significantly reduce the hardness of bread and improve the quality of bread.
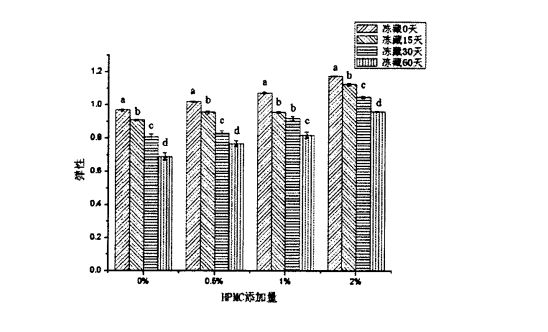
2.4 Mataupu Mataupu
Hydroxypropyl methylcellulose (HPMC) is a kind of hydrophilic colloid, and its application research in frozen dough with Chinese-style pasta food (such as steamed bread) as the final product is still lacking. The main purpose of this study is to evaluate the effect of HPMC improvement by investigating the effect of HPMC addition on the processing properties of frozen dough and the quality of steamed bread, so as to provide some theoretical support for the application of HPMC in steamed bread and other Chinese-style flour products. O iʻuga e faʻaalia ai o le HPMC e mafai ona faʻaleleia atili ai le taupulega o le paluga. When the addition amount of HPMC is 2%, the water absorption rate of the dough increases from 58.10% in the control group to 60.60%; 2 min increased to 12.2 min; at the same time, the dough formation time decreased from 2.1 min in the control group to 1.5 mill; the weakening degree decreased from 55 FU in the control group to 18 FU. In addition, HPMC also improved the tensile properties of the dough. With the increase in the amount of HPMC added, the elongation of the dough increased significantly; significantly reduced. In addition, during the frozen storage period, the addition of HPMC reduced the increase rate of the freezable water content in the dough, thereby inhibiting the damage to the dough network structure caused by ice crystallization, maintaining the relative stability of the dough viscoelasticity and the integrity of the network structure, thereby improving the stability of the dough network structure. The quality of the final product is guaranteed.
O le saito glitn o le sili ona tele o le teuina o le tinoitini According to the solubility of its components, it can be roughly divided into glutenin (soluble in alkaline solution) and gliadin (soluble in alkaline solution). in ethanol solution). Among them, the molecular weight (mw) of glutenin is as high as 1x107Da, and it has two subunits, which can form intermolecular and intramolecular disulfide bonds; while the molecular weight of gliadin is only 1x104Da, and there is only one subunit, which can form molecules Internal disulfide bond [100]. Campos, Steffe, & Ng (1 996) divided the formation of dough into two processes: energy input (mixing process with dough) and protein association (formation of dough network structure). It is generally believed that during dough formation, glutenin determines the elasticity and structural strength of the dough, while gliadin determines the viscosity and fluidity of the dough [102]. It can be seen that gluten protein has an indispensable and unique role in the formation of the dough network structure, and endows the dough with cohesion, viscoelasticity and water absorption.
3.2.1 Faʻataʻitaʻiga Mea
Glutun Ahuiru Rubi Fu XGIAG Food Co., Ltd.; Hydroxypropyl methylcelsulose (hpmc, tutusa pei o luga) Aladin vaifoma masani co., ltd.
DSC. Q200 Faʻaopoopo Scanning Calolometer
Jsm. 6490lv tungsten filament scanning engin newsonic microscope
Otometi Microplate Reader
Fd. 1b. 50 VIFUUM FREEZ MULIMULI
Tagata gaosi
Shanghai Spect Wards comboss co., LTD.
Jintin jincheng groosheng faʻataʻitaʻiga meafaigaluega
Thermo nicolet, USA
O vailaʻau uma o loʻo faʻaaogaina e faʻaaogaina i faʻataʻitaʻiga o le auiliiliga vasega.
3.2.4.2 Sauniga o le Frozen Droll Gluten paluga (gluten paluga)
3.2.4.3 Faʻamasinoga o tala o le susu o le susu susu
O le 15 mg faʻataʻitaʻiga o le susu o Gluten na mamafa ma faʻamaufaʻailogaina i se Aluminum Cructuble (talafeagai mo le suavai faʻataʻitaʻiga). The determination procedure and parameters are as follows: equilibrate at 20°C for 5 min, then drop to .30°C at a rate of 10°C/min, keep the temperature for 10 min, and finally increase to 25°C at a rate of 5°C/min, purge the gas (Purge Gas) was nitrogen (N2) and its flow rate was 50 mL/min, and a blank sealed aluminum crucible was used as a reference. The obtained DSC curve was analyzed using the analysis software Universal Analysis 2000, by analyzing the peaks located around 0 °C. Integral to get the melting enthalpy of ice crystals (Yu day). Then, the freezable water content (CFW) is calculated by the following formula [85-86]:
3.2.4.4.5 fuafuaina o Free Sulfhydryl mataupu (C) o saito Gluthen
Sodium sodium (sd). Tris-hydroxymethyl aminomethane (Tris). Glycine (Gly). Tetraacetic acid 7, amine (EDTA) buffer (10.4% Tris, 6.9 g glycine and 1.2 g EDTA/L, pH 8.0, abbreviated as TGE, and then 2.5% SDS It was added to the above TGE solution (that is, prepared into SDS-TGE buffer), incubated at 25°C for 30 min, and shaken every 10 min. Then, the supernatant was obtained after centrifugation for 10 min at 4°C and 5000×g. First, the protein content in the supernatant was determined by the Coomassie brilliant blue (G.250) method. Then, to the supernatant was added O. 04 mL of Ellman's reagent (dissolve 5,5'. Dithio-2. Nitrobenzoic acid, DTNB at TGE to measure the solution, 4 rag/ml), after 30 minutes of incubation in a 25 ℃ water bath, add 412 nm absorbance, and the above buffer was used as blank control. Finally, the free sulfhydryl content was calculated according to the following formula:
Among them, M is the function of the exponential decay sum of the signal amplitude with time (t) as the independent variable; Yang) o le galuega o le hydrogen proton numera numera ma le malologa taimi (d) pei o le tutoatasi suiga.
Using the CONTIN algorithm in the Provencher analysis software combined with the Laplace inverse transformation, the inversion is performed to obtain a continuous distribution curve. O faʻataʻitaʻiga taʻitasi na toe faia faʻalua taimi
In this experiment, a Fourier transform infrared spectrometer equipped with an attenuated single reflection attenuated total reflection (ATR) accessory was used to determine the secondary structure of gluten protein, and a cadmium mercury telluride crystal was used as the detector. Uma le faʻataʻitaʻiga ma talaʻaga o loʻo siakiina 64 taimi ma se iugafono o le 4 cm ~ ma le scansing i le 4000 cmq-500 cm ~. Spread a small amount of protein solid powder on the surface of the diamond on the ATR fitting, and then, after 3 turns clockwise, you can start to collect the infrared spectrum signal of the sample, and finally get the wavenumber (Wavenumber, cm-1) as the abscissa, and absorbance as the abscissa. (Absorption) is the infrared spectrum of the ordinate.
All results are expressed as mean 4-standard deviation, and the above experiments were repeated at least three times except for scanning electron microscopy. Faʻaaoga le amataga 8.0 e tusi ai siata, ma faʻaaoga spss 19.0 mo le tasi. Way analysis of variance and Duncan's multiple range test, the significance level was 0.05.
3. Faʻaiʻuga ma talanoaga
3.3.1 Aafiaga o le HPMC SECMC SECELE MA LE FUA MA LE FOLAFOLAGA O LE TAIMI TAIMI I LE DHEUMAL PRUMENT O SMO GLOUNS
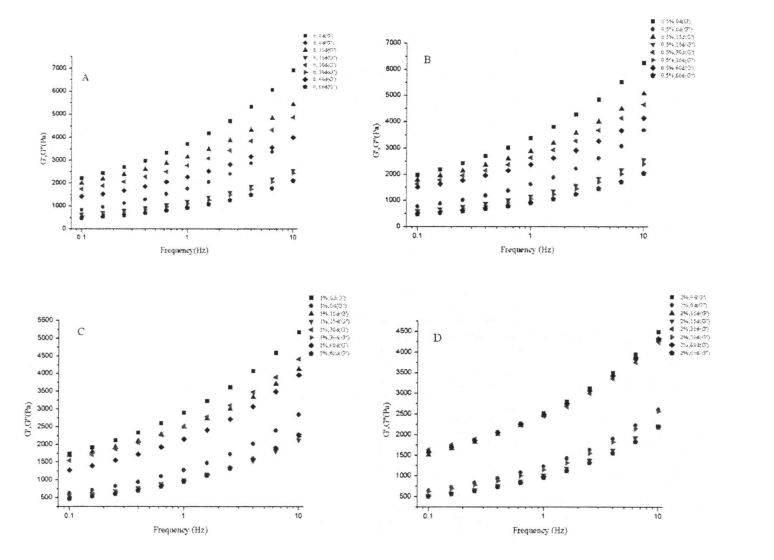
Ata 3.1 Aafiaga o HPMC Screem ma Frozen Teuina i luga o le tala fatu o Gluten paluga
3.3.2 Aafiaga o le HPMC Seam Seam ma le faʻaʻaisa o le teuina taimi i luga o le 'aisa susu meatama (CFW) ma le le atoatoa o le soifua maloloina
3.3.2.2.2 FAAMOEMOI O LE FAʻAVAVAU O MEA O LE HPMC MA LE FAʻAFETAI O LE FAAFITAULI O LE HALAMAL LE HALAMAL O LE PLUTUNC Porotin
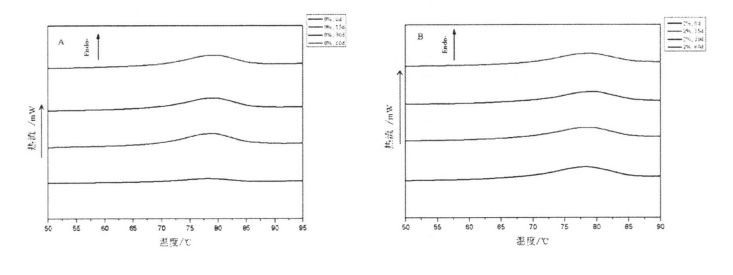
The thermal stability of gluten has an important influence on the grain formation and product quality of thermally processed pasta [211]. Figure 3.2 shows the obtained DSC curve with temperature (°C) as the abscissa and heat flow (mW) as the ordinate. The experimental results (Table 3.3) found that the heat denaturation temperature of gluten protein without freezing and without adding I-IPMC was 52.95 °C, which was consistent with Leon, et a1. (2003) and Khatkar, Barak, & Mudgil (2013) reported very similar results [120m11. With the addition of 0% unfrozen, O. Compared with the heat denaturation temperature of gluten protein with 5%, 1% and 2% HPMC, the heat deformation temperature of gluten protein corresponding to 60 days increased by 7.40℃, 6.15℃, 5.02℃ and 4.58℃, respectively. Obviously, under the condition of the same freezing storage time, the increase of denaturation peak temperature (N) decreased sequentially with the increase of HPMC addition. This is consistent with the change rule of the results of Cry. I se faʻaopopoga, mo faʻataʻitaʻiga ulo, aʻo faʻalauteleina le tele o le HPMC, o le faʻata o le tupe faʻaalu. This may be due to the intermolecular interactions between HPMC with molecular surface activity and gluten, such as the formation of covalent and non-covalent bonds [122J].
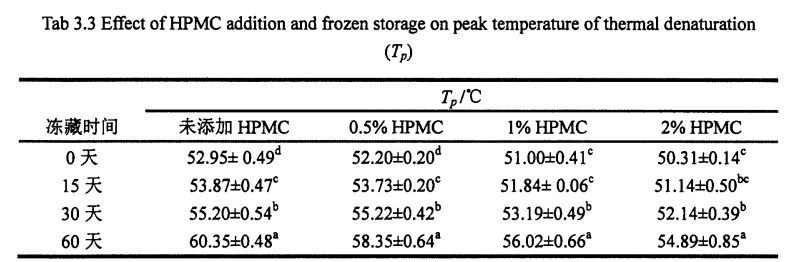
Fig 3.2 Typical DSC thermograms of gluten proteins with 0%HPMC(A);with O.5%HPMC(B); with 1%HPMC(C);with 2%HPMC(D)after different time of frozen storage,from 0d to 60d indicated from the lowest curve to the highest one in each graph. FAAMATALAGA: O le DSC curve o le saito glutn e aunoa ma le faaopoopoina o HPMC; B o le faʻaopopoina o le O. DSS Curve o saito Glutun ma le 5% HPMC; C o le DSC Curve o saito Glutun ma 1% HPMC; D is the DSC curve of wheat gluten with 2% HPMC 3.3.3 Effects of HPMC addition amount and freezing time on free sulfhydryl content (C-SH) Intermolecular and intramolecular covalent bonds are very important for the stability of dough network structure. A disulfide bond (-SS-) is a covalent linkage formed by dehydrogenation of two free sulfhydryl groups (.SH). Glutenin is composed of glutenin and gliadin, the former can form intramolecular and intermolecular disulfide bonds, while the latter can only form intramolecular disulfide bonds [1241] Therefore, disulfide bonds are an intramolecular/intermolecular disulfide bond. important way of cross-linking. Compared to adding 0%, O. The C-SH of 5% and 1% HPMC without freezing treatment and the C-SH of gluten after 60 days of freezing have different degrees of increase respectively. Specifically, the face with no HPMC added gluten C. SH increased by 3.74 "mol/g to 8.25 "mol/g, while C.sh, shellfish, with gluten supplemented with 0.5% and 1% HPMC increased by 2.76 "mol/g to 7.25""mol/g and 1.33 "mol/g to 5.66 "mol/g (Fig. 3.3). Zhao, et a1. (2012) found that after 120 days of frozen storage, the content of free thiol groups increased significantly [ 1071. It is worth noting that the C-SH of gluten protein was significantly lower than that of other frozen storage periods when the freezing period was 15 days, which may be attributed to the freezing shrinkage effect of gluten protein structure, which makes the More intermolecular and intramolecular disulfide bonds were locally formed in a shorter freezing time [1161. Wang, et a1. (2014) found that the C-SH of glutenin-rich proteins was also significantly increased after 15 days of freezing. Decreased [1251. However, the gluten protein supplemented with 2% HPMC did not increase significantly except for C-SH, which also decreased significantly at 15 days, with the extension of freezing time.
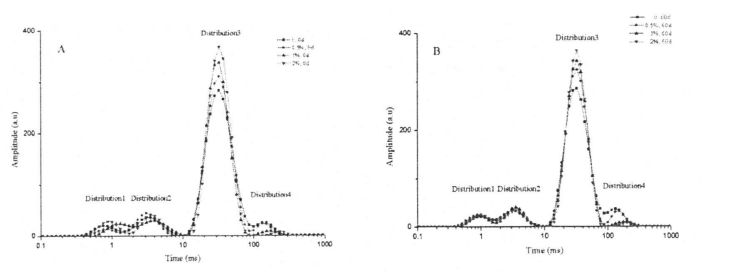
However, there were obvious differences in the height and area of T23 distribution of wet gluten mass that was not frozen and contained different HPMC additions, and with the increase of addition, the height and area of T23 distribution increased (Fig. 3.4). This change shows that HPMC can significantly increase the relative content of limited water, and it is positively correlated with the added amount within a certain range. In addition, with the extension of freezing storage time, the height and area of T23 distribution of the wet gluten mass with the same HPMC content decreased to varying degrees. Therefore, compared with bound water, limited water showed a certain effect on freezing storage. Mafaufau loloto. This trend suggests that the interaction between the gluten protein matrix and the confined water becomes weaker. This may be because more hydrophobic groups are exposed during freezing, which is consistent with the thermal denaturation peak temperature measurements. In particular, the height and area of the T23 distribution for the wet gluten mass with 2% HPMC addition did not show a significant difference. This indicates that HPMC can limit the migration and redistribution of water, and can inhibit the transformation of the water state from the restricted state to the free state during the freezing process.
Fourier transform infrared spectroscopy (FT-IR) has been widely used for high-throughput determination of the secondary structure of protein samples. The characteristic bands in the infrared spectrum of proteins mainly include, amide I band (1700.1600 cm-1), amide II band (1600.1500 cm-1) and amide III band (1350.1200 cm-1). Correspondingly, the amide I band the absorption peak originates from the stretching vibration of the carbonyl group (-C=O-.), the amide II band is mainly due to the bending vibration of the amino group (-NH-) [1271], and the amide III band is mainly due to the amino bending vibration and .CN-.Synchronous compound vibration in the same plane of bond stretching gatete, ma e iai le maualuga o le aoga i suiga i le poveina o le vaega lona lua [128'1291. Although the above three characteristic bands are all characteristic infrared absorption peaks of proteins, the specific In other words, the absorption intensity of amide II band is lower, so the semi-quantitative accuracy of protein secondary structure is poor; while the peak absorption intensity of amide I band is higher, so many researchers analyze the secondary structure of protein by this band [ 1301, but the absorption peak of water and the amide I band are overlapped at about 1640 cm. 1 wavenumber (Overlapped), which in turn affects the accuracy of the results. O le mea lea, o le faʻalavelave e faʻatapulaʻaina vai e faʻatapulaʻaina le uati o le aʻi i le poʻo le faʻamasinoga tulaga lua o loʻo fuafuaina. In this experiment, in order to avoid the interference of water, the relative contents of four secondary structures of gluten protein were obtained by analyzing the amide III band. Peak position (wavenumber interval) of
O le faʻauigaina ma faʻamaumauga o loʻo lisiina i le Lisi 3.4.
Tab 3.4 Peak Station ma le tofiga o tulaga lua fausaga na tupu mai i le AMD III BROW I FT-IR SPECTRA
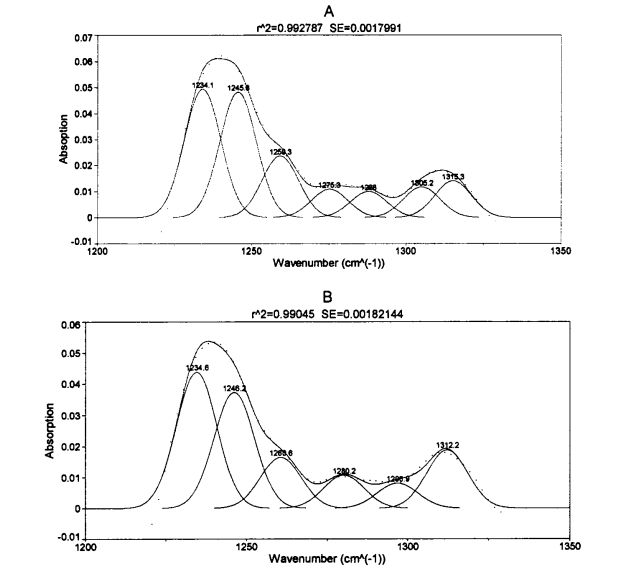
FAAMATALAGA: O le a le faʻaoʻoina o le chatat o saito glitun porotini e aunoa ma le faʻaopopoina o le HPMC mo 0 aso o loʻo teuina ai le teuina o le aisa; B o le faʻaʻaiina o le chatat o saito glitun poronitini o le teuina o aisa teu mo 0 aso ma le 2% hpmc faaopoopo
With the prolongation of frozen storage time, the secondary structure of gluten protein with different additions of HPMC changed to different degrees. It can be seen that both frozen storage and addition of HPMC have an effect on the secondary structure of gluten protein. Regardless of the amount of HPMC added, B. The folded structure is the most dominant structure, accounting for about 60%. After 60 days of frozen storage, add 0%, OB Gluten of 5% and 1% HPMC. The relative content of folds increased significantly by 3.66%, 1.87% and 1.16%, respectively, which was similar to the results determined by Meziani et al. (2011) [L33J]. Peitaʻi, e leai se eseesega tele i le taimi o le faʻaʻaisa o le faʻaputuga mo glutn sueina ma le 2% hpmc. E le gata i lea, pe a pa 'aisa mo 0 aso, ma le faʻateleina o le HPMC faʻaopopo, i. O le taulealea aano o le foeina i luga teisi teisi, aemaise lava pe a le taua le aofaiga sa 2%, i. O le taulealea meatotino o loʻo siʻitia i le 2.01%. O le ata o le ata e mafai ona vaevaeina i le symolecular p. Gagau (mafua mai i le tuufaatasiga o porotini molecles), antpafarallalel p. Folded and parallel p. Three substructures are folded, and it is difficult to determine which substructure occurs during the freezing process
suia. Some researchers believe that the increase in the relative content of the B-type structure will lead to an increase in the rigidity and hydrophobicity of the steric conformation [41], and other researchers believe that p. The increase in folded structure is due to part of the new β-Fold formation is accompanied by a weakening of the structural strength maintained by hydrogen bonding [421]. β- The increase in the folded structure indicates that the protein is polymerized through hydrophobic bonds, which is consistent with the results of the peak temperature of thermal denaturation measured by DSC and the distribution of transverse relaxation time measured by low-field nuclear magnetic resonance. Detand i le deotan. On the other hand, added 0.5%, 1% and 2% HPMC gluten protein α-whirling. The relative content of helix increased by 0.95%, 4.42% and 2.03% respectively with the prolongation of freezing time, which is consistent with Wang, et a1. (2014) found similar results [134]. 0 of gluten without added HPMC. There was no significant change in the relative content of helix during the frozen storage process, but with the increase of the addition amount of freeze for 0 days. There were significant differences in the relative content of α-whirling structures.
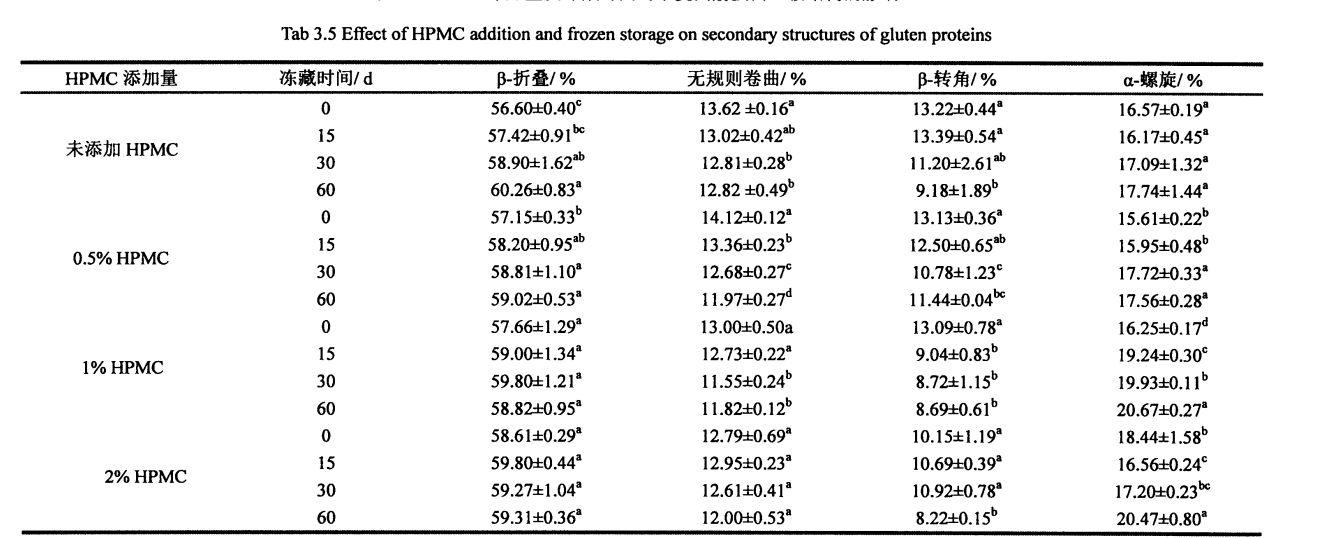
O faʻataʻitaʻiga uma i le faʻalauteleina o le taimi faʻaʻaisa, i. The relative contents of the corners were significantly reduced. This shows that β-turn is very sensitive to freezing treatment [135. 1361], and whether HPMC is added or not has no effect. Manuia, et a1. (2005) proposed that the β-chain turn of gluten protein is related to the β-turn space domain structure of the glutenin polypeptide chain [l 37]. Except that the relative content of random coil structure of gluten protein added with 2% HPMC had no significant change in frozen storage, the other samples were significantly reduced, which may be caused by the extrusion of ice crystals. In addition, when frozen for 0 days, the relative contents of α-helix, β-sheet and β-turn structure of gluten protein added with 2% HPMC were significantly different from those of gluten protein without HPMC. This may indicate that there is an interaction between HPMC and gluten protein, forming new hydrogen bonds and then affecting the conformation of the protein; or HPMC absorbs the water in the pore cavity of the protein space structure, which deforms the protein and leads to more changes between the subunits. close. The increase of the relative content of β-sheet structure and the decrease of the relative content of β-turn and α-helix structure are consistent with the above speculation. During the freezing process, the diffusion and migration of water and the formation of ice crystals destroy the hydrogen bonds that maintain the conformational stability and expose the hydrophobic groups of proteins. In addition, from the perspective of energy, the smaller the energy of the protein, the more stable it is. At low temperature, the self-organization behavior (folding and unfolding) of protein molecules proceeds spontaneously and leads to conformational changes.
3.3.6 Aafiaga o le HPMC Faaopopo Aofaiga ma le faʻaʻaisa o le teuina taimi i luga o le hydrophobicity o Glutun Porotini
O le porotini moctor e aofia ai uma hydrophilic ma hydrophobic vaega. Generally, the protein surface is composed of hydrophilic groups, which can bind water through hydrogen bonding to form a hydration layer to prevent protein molecules from agglomerating and maintain their conformational stability. The interior of the protein contains more hydrophobic groups to form and maintain the secondary and tertiary structure of the protein through the hydrophobic force. Denaturation of proteins is often accompanied by exposure of hydrophobic groups and increased surface hydrophobicity.

Eseʻese mataitusi lapopoa mataitusi i totonu o le koluma lava e tasi e faʻaalia ai le taua tele (<0.05);
3.3.7 Aafiaga o HPMC SECMC SEFE LE VAEGA MA LE FOLAFOLAINA LE TAIMI TAIMI TAIMI I LE MINI-FESOASOANI FAIGA O GLLITEN
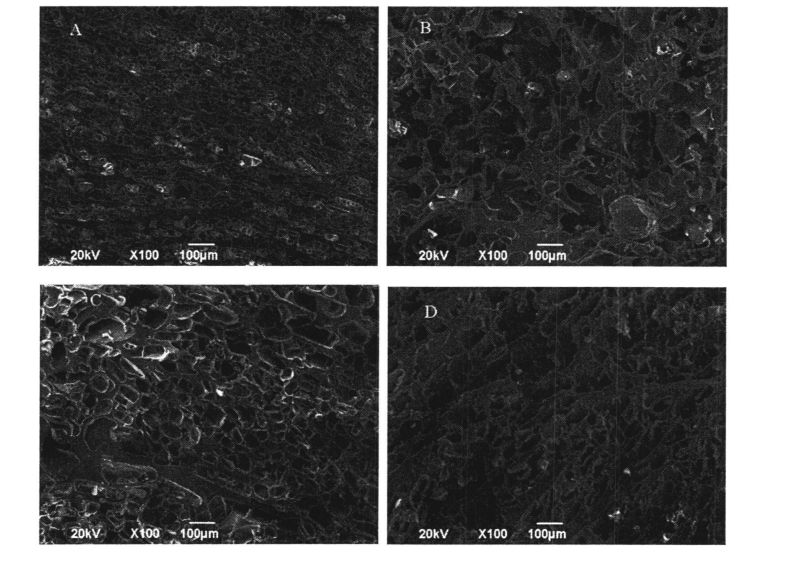
FAAMATALAGA: A o le abrastrgerrrrget of Gluten Network e aunoa ma le faʻaopopoina o le HPMC ma faʻaʻaisa mo 0 aso; B is the microstructure of gluten network without adding HPMC and frozen for 60 days; C is the microstructure of gluten network with 2% HPMC added and frozen for 0 days :D is the gluten network microstructure with 2% HPMC added and frozen for 60 days
After 60 days of frozen storage, the microstructure of the wet gluten mass without HPMC was significantly changed (Fig. 3.7, AB). I le 0 aso, o le glutsed bargetries ma le 2% poʻo le 0% hpmc faʻaalia atoatoa foliga atoatoa, tele
3.4 Mataupu Mataupu
This experiment investigated the rheology of wet gluten dough and gluten protein by adding HPMC with different contents (0%, 0.5%, 1% and 2%) during freezing storage (0, 15, 30 and 60 days). meatotino, meatotino a le Hormadynimis, ma le aafiaga o le Hotecoochomical meatotino. The study found that the change and redistribution of water state during the freezing storage process significantly increased the freezable water content in the wet gluten system, which led to the destruction of the gluten structure due to the formation and growth of ice crystals, and ultimately caused the processing properties of the dough to be different. Deterioration of product quality. The results of frequency scanning showed that the elastic modulus and viscous modulus of the wet gluten mass without adding HPMC decreased significantly during the freezing storage process, and the scanning electron microscope showed that its microstructure was damaged. The content of free sulfhydryl group was significantly increased, and its hydrophobic group was more exposed, which made the thermal denaturation temperature and surface hydrophobicity of gluten protein significantly increased. However, the experimental results show that the addition of I-IPMC can effectively inhibit the changes in the structure and properties of wet gluten mass and gluten protein during freezing storage, and within a certain range, this inhibitory effect is positively correlated with the addition of HPMC. This is because HPMC can reduce the mobility of water and limit the increase of the freezable water content, thereby inhibiting the recrystallization phenomenon and keeping the gluten network structure and the spatial conformation of the protein relatively stable. This shows that the addition of HPMC can effectively maintain the integrity of the frozen dough structure, thereby ensuring product quality.
MATAUPU 4 Aafiaga o HPMC Suesegaina o le fausaga ma meatotino a le starch i lalo o le aisa teu
Starch is one of the main components of flour, and its content is as high as about 75% (dry basis). I le taimi lava e tasi, o se avetatala lautele i totonu o fatu, o le Starte o le autu lea o le malosi ono fua i meaai. In the dough system, starch is mostly distributed and attached to the network structure of gluten protein. During processing and storage, starches often undergo gelatinization and aging stages.
Among them, starch gelatinization refers to the process in which starch granules are gradually disintegrated and hydrated in a system with high water content and under heating conditions. It can be roughly divided into three main processes. 1) Reversible water absorption stage; before reaching the initial temperature of gelatinization, the starch granules in the starch suspension (Slurry) keep their unique structure unchanged, and the external shape and internal structure basically do not change. Only very little soluble starch is dispersed in the water and can be restored to its original state. 2) The irreversible water absorption stage; as the temperature increases, water enters the gap between the starch crystallite bundles, irreversibly absorbs a large amount of water, causing the starch to swell, the volume expands several times, and the hydrogen bonds between the starch molecules are broken. It becomes stretched and the crystals disappear. At the same time, the birefringence phenomenon of starch, that is, the Maltese Cross observed under a polarizing microscope, begins to disappear, and the temperature at this time is called the initial gelatinization temperature of starch. 3) Starcpy Granile Detinication Station; starch molecules completely enter the solution system to form starch paste (Paste/Starch Gel), at this time the viscosity of the system is the largest, and the birefringence phenomenon completely disappears, and the temperature at this time is called the complete starch gelatinization temperature, the gelatinized starch is also called α-starch [141]. When the dough is cooked, the gelatinization of starch endows the food with its unique texture, flavor, taste, color, and processing characteristics.
4.2.1 Faʻataʻitaʻiga mea
WHAD STARCH BINZHOU ZHENGYU FOʻI FOʻI FO., ltd.; HPMC Aladdin (Shanghai) vailaau Reagent Co., Ltd.;
BC / BD-272 Swrigearator
Mauaina R3 Rourtional Rheometer
D / Max2500v ituaiga X. Ray Raractocameter
Tagata gaosi
Hier vaega
Ahui Zhongke Zhongjia Tusi faasaienisi Pretics co., ltd.
In this experiment, a rheometer was used instead of a fast viscometer to measure the gelatinization characteristics of starch. Vaai bae et a1. (2014) metotia [1571] ma nai fesuiaiga. The specific program parameters are set as follows: use a plate with a diameter of 40 mill, the gap (gap) is 1000 mm, and the rotation speed is 5 rad/s; I) incubate at 50 °C for 1 min; II) i le 5. C / MULIMULI I LE 95 ° C; iii) kept at 95°C for 2.5 min, iv) then cooled to 50°C at 5°C/min; v) lastly held at 50°C for 5 min.
Ave 2.5 g o Amyloid ma faʻafefiloi ma le malulu vai i se fua faatatau o le 1: 2 e faia ai le pato susu. Freeze at 18°C for 15 d, 30 d, and 60 d. Faʻaopopo 0.5, 1, 2% HPMC (W / W) e suia le tulaga tutusa o le tulaga tutusa, ma isi sauniuniga e le suia. After the freezing treatment is completed, take it out, equilibrate at 4 °C for 4 h, and then thaw at room temperature until it is tested.
(3) stess gel malosi (gel malosi)
4.2.3.4 o mea a le Hormadynamic
Ina ua maeʻa le taimi vaʻaia o le mataʻutia, o faʻataʻitaʻiga na ave ese, sa oso aʻe atoa, ma mago i se ogaumu i le 40 ° C mo le 48 h. Finally, it was ground through a 100-mesh sieve to obtain a solid powder sample for use (suitable for XRD testing). Vaʻai le xie, et a1. (2014) method for sample preparation and determination of thermodynamic properties '1611, weigh 10 mg of starch sample into a liquid aluminum crucible with an ultra-micro analytical balance, add 20 mg of distilled water in a ratio of 1:2, press and seal it and place it at 4 °C In the refrigerator, equilibrated for 24 h. Freeze at 18°C (0, 15, 30 and 60 days). Add 0.5%, 1%, 2% (w/w) HPMC to replace the corresponding quality of starch, and other preparation methods remain unchanged. After the freezing storage time is over, take out the crucible and equilibrate at 4 °C for 4 h.
The thawed frozen starch samples were dried in an oven at 40 °C for 48 h, then ground and sieved through a 100-mesh sieve to obtain starch powder samples. Take a certain amount of the above samples, use D/MAX 2500V type X. The crystal form and relative crystallinity were determined by X-ray diffractometer. O le faʻataʻitaʻiga tulaga o loʻo voltage 40 KV, o le taimi nei 40 ma, faʻaaogaina Cu. Ks as X. ray source. At room temperature, the scanning angle range is 30--400, and the scanning rate is 20/min. Relative crystallinity (%) = crystallization peak area/total area x 100%, where the total area is the sum of the background area and the peak integral area [1 62].
4.2.3.6.6 Fuafuaina o Starch Blue
4.2.3.7 Faʻamatalaga o Faʻamatalaga ma Faʻagaioiga
All experiments were repeated at least three times unless otherwise specified, and the experimental results were expressed as mean and standard deviation. SPSS Statistics 19 sa faʻaaogaina mo le auiliiliga o le eseese (auiliiliga o le eseese, arva) ma le taua tulaga o 0.05; O siata o loʻo tusia ai ni mea e oʻo mai ai 8.0.
4.3 auiliiliga ma talanoaga
According to GB 50093.2010, GB/T 5009.9.2008, GB 50094.2010 (78-s0), the basic components of wheat starch - moisture, amylose/amylopectin and ash content were determined. O iʻuga o loʻo faʻaalia i le Lisi 4. 1 faʻaalia.
Maua le 4.1 mataupu o le aofia ai o saito

The starch suspension with a certain concentration is heated at a certain heating rate to make the starch gelatinized. After starting to gelatinize, the turbid liquid gradually becomes pasty due to the expansion of starch, and the viscosity increases continuously. Subsequently, the starch granules rupture and the viscosity decreases. A malulu le palu i se fua mautu, o le palu o le a susulu mai ai le palu, ma le vicoscom o le a faʻateleina atili ai. Le viscosity aoga pe a malulu i le 50 ° C o le tau mulimuli o le vircomaty tau (Ata 4.1).
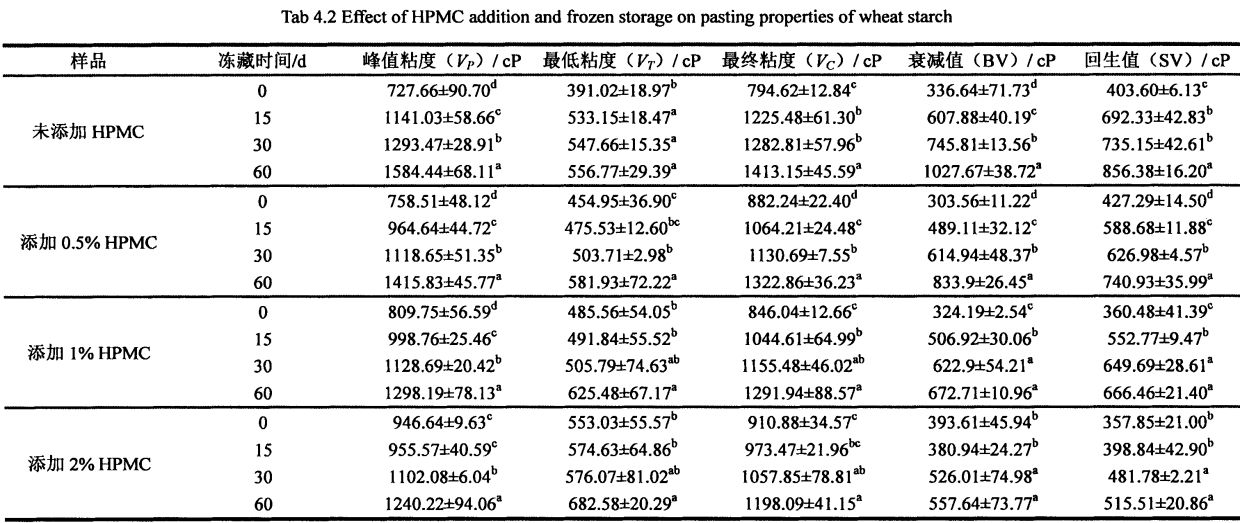
P (Frozen Teuina mo 0 Aso) i le 856.38 ± 16.20 CP (Inronzen Teu mo 60 aso); the retrogradation value of starch suspension added with 0.5% HPMC increased from 427 .29±14.50 CP (frozen storage for 0 days) increased to 740.93±35.99 CP (frozen storage for 60 days); O le toe faʻaleleia o le tau o le starc desapepen faʻaopopo ma 1% hpmc faʻateleina mai le 360.48 ± 41. 39 CP (frozen storage for 0 days) increased to 666.46 ± 21.40 CP (frozen storage for 60 days); while the retrogradation value of starch suspension added with 2% HPMC increased from 357.85 ± 21.00 CP (frozen storage for 60 days). 0 days) increased to 515.51 ± 20.86 CP (60 days frozen).
It can be seen that with the prolongation of freezing storage time, the starch gelatinization characteristics index increased, which is consistent with Tao et a1. f2015) 1. Consistent with the experimental results, they found that with the increase of the number of freeze-thaw cycles, the peak viscosity, minimum viscosity, final viscosity, decay value, and retrogradation value of starch gelatinization all increased to different degrees [166J]. This is mainly because in the process of freezing storage, the amorphous region (Amorphous Region) of starch granules is destroyed by ice crystallization, so that the amylose (the main component) in the amorphous region (non-crystalline region) undergoes phase separation (Phase. separated) phenomenon, and dispersed in the starch suspension, resulting in an increase in the viscosity of starch gelatinization, and an increase in the related attenuation value and retrogradation value. However, the addition of HPMC inhibited the effect of ice crystallization on starch structure. Therefore, the peak viscosity, minimum viscosity, final viscosity, decay value and retrogradation rate of starch gelatinization increased with the addition of HPMC during frozen storage. faateleina ma le faaitiitia o le vave.
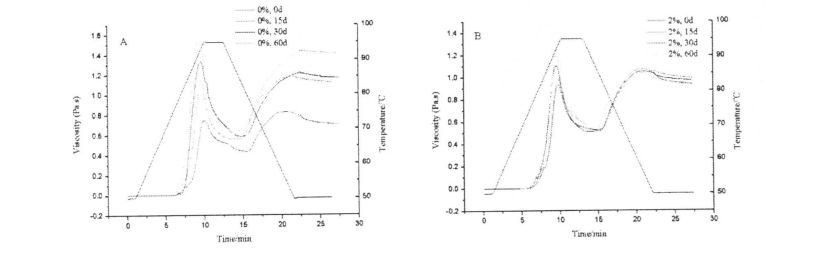
4.33.3 Aafiaga o le HPMC Seam Seam ma Badzen Teu Taimi i le Shear Vercosity o Starch Platte
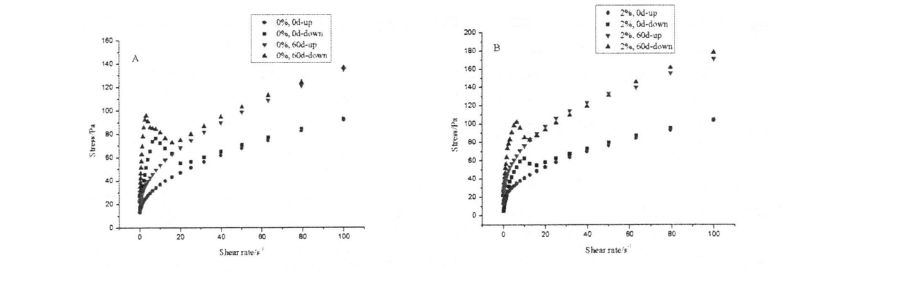
Fig 4.2 Thixotropism o Stars Paste e aunoa ma HPMC (A) poʻo le 2% HPMC (B)
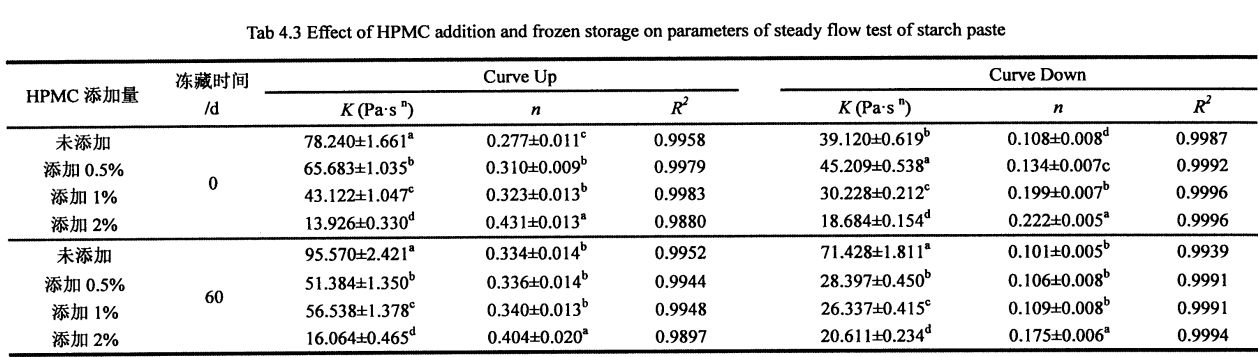
It can be seen from Table 4.3 that all the flow characteristic indices, 2, are less than 1. Therefore, starch paste (whether HPMC is added or whether it is frozen or not) belongs to Pseudoplastic Fluid, and all show shearing Thinning phenomenon (as the shear rate increases, the shear viscosity of the fluid decreases). In addition, the shear rate scans ranged from 0.1 s, respectively. 1 increased to 100 s ~, and then decreased from 100 sd to O. The rheological curves obtained at 1 sd do not completely overlap, and the fitting results of K, s are also different, so the starch paste is a thixotropic pseudoplastic fluid (whether HPMC is added or whether it is frozen or not). However, under the same freezing storage time, with the increase of HPMC addition, the difference between the fitting results of the K n values of the two scans gradually decreased, which indicates that the addition of HPMC makes the structure of starch paste under shear stress. E tumau pea le tumau i lalo o le gaioiga ma faʻaititia le "Tyxotropic mama"
(Thixotropic Loop) area, which is similar to Temsiripong, et a1. (2005) lipotia le faaiuga lava e tasi [167]. This may be mainly because HPMC can form intermolecular cross-links with gelatinized starch chains (mainly amylose chains), which "bound" the separation of amylose and amylopectin under the action of shearing force. , so as to maintain the relative stability and uniformity of the structure (Figure 4.2, the curve with shear rate as abscissa and shear stress as ordinate).
However, with the prolongation of freezing storage time, the values of K and n increased to different degrees, among which the value of K increased from 78.240 ± 1.661 Pa·sn (unadded, 0 days) to 95.570 ± 1, respectively. 2.421 Pa·sn (no addition, 60 days), increased from 65.683±1.035 Pa ·S n (addition of O. 5% HPMC, 0 days) to 51.384±1.350 Pa ·S n (Add to 0.5% HPMC, 60 days), increased from 43.122±1.047 Pa ·sn (adding 1% HPMC, 0 days) to 56.538±1.378 Pa ·sn (adding 1% HPMC, 60 days) ), and increased from 13.926 ± 0.330 Pa ·sn (adding 2% HPMC, 0 days) to 16.064 ± 0.465 Pa ·sn (adding 2% HPMC, 60 days); 0.277 ± 0.011 (without adding HPMC, 0 days) rose to O. 334±0.014 (no addition, 60 days), increased from 0.310±0.009 (0.5% HPMC added, 0 day) to 0.336±0.014 (0.5% HPMC added, 60 days), from 0.323 ± 0.013 (add 1% HPMC, 0 days) to 0.340 ± 0.013 (add 1% HPMC, 60 days), and from 0.431 ± 0.013 (add 1% HPMC, 60 days) 2% HPMC, 0 days) to 0.404+0.020 (add 2% HPMC, 60 days). By comparison, it can be found that with the increase of the addition amount of HPMC, the change rate of K and Knife value decreases successively, which shows that the addition of HPMC can make the starch paste stable under the action of shearing force, which is consistent with the measurement results of starch gelatinization characteristics. faifai pea.
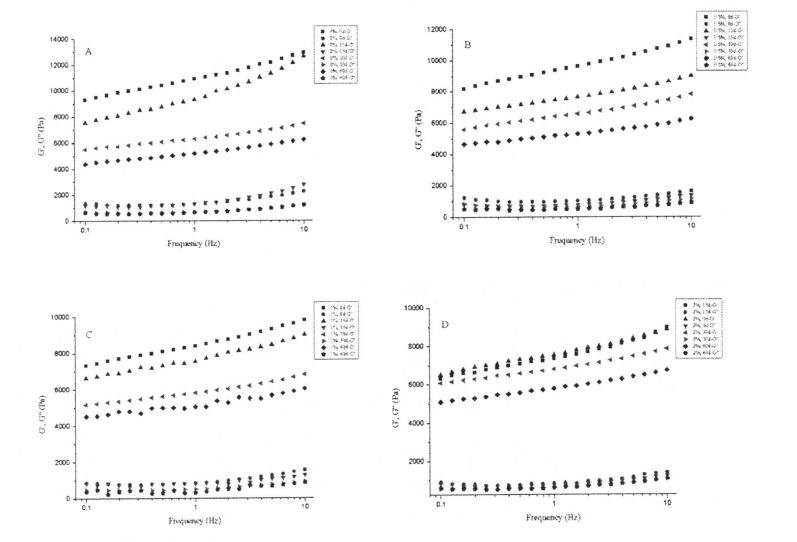
Fig 4.3 aafiaga o le HPMC SET Sweet ma Frozen Teuina i le Elastic ma Viscous Addious o Starch Prete
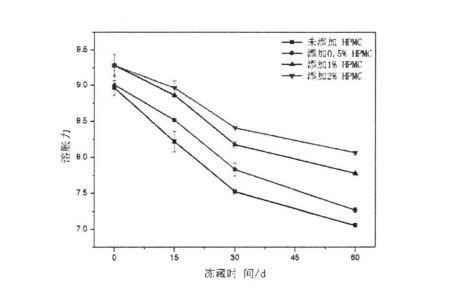
O le Fig 4.4 Faʻamanuiaina o le HPMC Screem ma Frozen Teuina i luga o le fulafula le malosi o le starch
4.3.6 Aafiaga o le HPMC Seam Mat o le Away ma Frozen Teuina Taimi i luga o le Hermodicynamic meatotino o le starch
As shown in Table 4.4, for fresh amyloid, with the increase of HPMC addition, starch L has no significant difference, but increases significantly, from 77.530 ± 0.028 (without adding HPMC) to 78.010 ± 0.042 (add 0.5% HPMC), 78.507 ± 0.051 (add 1% HPMC), and 78.606 ± 0.034 (add 2% HPMC), but 4H is significant Decrease, from 9.450 ± 0.095 (without adding HPMC) to 8.53 ± 0.030 (adding 0.5% HPMC), 8.242A: 0.080 (adding 1% HPMC) and 7 .736 ± 0.066 (add 2% HPMC). E tutusa lea ma le Zhou, et a1. (2008) found that adding a hydrophilic colloid decreased the starch gelatinization enthalpy and increased the starch gelatinization peak temperature [172]. This is mainly because HPMC has better hydrophilicity and is easier to combine with water than starch. At the same time, due to the large temperature range of the thermally accelerated gelation process of HPMC, the addition of HPMC increases the peak gelatinization temperature of starch, while the gelatinization Enthalpy decreases.
However, with the prolongation of freezing storage time, the relative crystallinity of starch increased from 20.40 + 0.14 (without HPMC, 0 days) to 36.50 ± 0.42 (without HPMC, frozen storage, respectively). 60 days), and increased from 25.75 + 0.21 (2% HPMC added, 0 days) to 32.70 ± 0.14 (2% HPMC added, 60 days) (Figure 4.6.B), this and Tao, et a1. (2016), Ole suiga ole tulafono ole fua e tutusa ile [1774]. The increase in relative crystallinity is mainly caused by the destruction of the amorphous region and the increase in the crystallinity of the crystalline region. In addition, consistent with the conclusion of the changes in the thermodynamic properties of starch gelatinization, the addition of HPMC reduced the degree of relative crystallinity increase, which indicated that during the freezing process, HPMC could effectively inhibit the structural damage of starch by ice crystals and maintain the Its structure and properties are relatively stable.
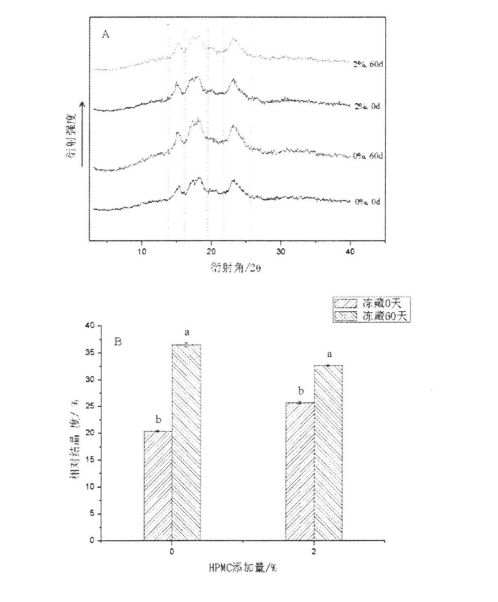
Note: A is x. X-Ray tulaga ese; E e na te le mafuʻea le taua o le turch;
Yeast has a wide range of applications in fermented flour products (sourdough is obtained by natural fermentation, mainly lactic acid bacteria), it can use the hydrolyzed product of starch in the dough - glucose or maltose as a carbon source, under aerobic conditions, using Substances produce carbon dioxide and water after respiration. O le kapeponi dioxide gaosia e mafai ona faia le paluga le paluga, sasaina ma le tele. At the same time, the fermentation of yeast and its role as an edible strain can not only improve the nutritional value of the product, but also significantly improve the flavor characteristics of the product. Therefore, the survival rate and fermentation activity of yeast have an important impact on the quality of the final product (specific volume, texture, and flavor, etc.) [175].
In the case of frozen storage, yeast will be affected by environmental stress and affect its viability. When the freezing rate is too high, the water in the system will rapidly crystallize and increase the external osmotic pressure of the yeast, thereby causing the cells to lose water; A maualuga le fua o le fua faatatau. If it is too low, the ice crystals will be too large and the yeast will be squeezed and the cell wall will be damaged; both will reduce the survival rate of the yeast and its fermentation activity. In addition, many studies have found that after the yeast cells are ruptured due to freezing, they will release a reducing substance-reduced glutathione, which in turn reduces the disulfide bond to a sulfhydryl group, which will eventually destroy the network structure of gluten protein, resulting in a decrease in the quality of pasta products [176-177].
3m malosi ata tifaga clony cread faitauga suega
Zwy-240 tumau vevela vevela
Tagata gaosi
Ahui Zhongke Zhongjia Tusi faasaienisi Pretics co., ltd.
Shanghai Zhichheng Assictical meafaigaluega gaosiga co., ltd.
See Meziani, et a1. (2012)'s experimental method [17 cited, with slight modifications. Weigh 5 g of frozen dough into a 50 mL colorimetric tube, press the dough to a uniform height of 1.5 cm at the bottom of the tube, then place it upright in a constant temperature and humidity box, and incubate for 1 h at 30 °C and 85% RH, after taking it out, measure the proofing height of the dough with a millimeter ruler (retain two digits after the decimal point). For samples with uneven upper ends after proofing, select 3 or 4 points at equal intervals to measure their corresponding heights (for example, each 900), and the measured height values were averaged. O faʻataʻitaʻiga taʻitasi na fetuunai faatolu.
5.2.2.3 CFU (Colony-Faia iunite) faitau
Weigh 1 g of dough, add it to a test tube with 9 mL of sterile normal saline according to the requirements of the aseptic operation, shake it fully, record the concentration gradient as 101, and then dilute it into a series of concentration gradients until 10'1. Draw 1 mL of dilution from each of the above tubes, add it to the center of the 3M yeast rapid count test piece (with strain selectivity), and place the above test piece in a 25°C incubator according to the operating requirements and culture conditions specified by 3M. 5 d, take out after the end of the culture, first observe the colony morphology to determine whether it conforms to the colony characteristics of yeast, and then count and microscopically examine [179]. Sa taʻutaʻu atu faʻataʻitaʻiga i le tolu taimi.
The alloxan method was used to determine the glutathione content. The principle is that the reaction product of glutathione and alloxan has an absorption peak at 305 nl. Specific determination method: pipette 5 mL of yeast solution into a 10 mL centrifuge tube, then centrifuge at 3000 rpm for 10 min, take 1 mL of supernatant into a 10 mL centrifuge tube, add 1 mL of 0.1 mol/mL to the tube L alloxan solution, mixed thoroughly, then add 0.2 M PBS (pH 7.5) and 1 mL of 0.1 M, NaOH solution to it, mix well, let stand for 6 min, and immediately add 1 M, NaOH The solution was 1 mL, and the absorbance at 305 nm was measured with a UV spectrophotometer after thorough mixing. O le Glitathones mataupu na fuafuaina mai le laina tulaga faatonuina. O faʻataʻitaʻiga taʻitasi na fetuunai faatolu.
Experimental results are presented as 4-standard deviation of the mean, and each experiment was repeated at least three times. O le auiliiliga o fesuiaʻiga sa faia e faʻaaoga ai spss, ma le tulaga taua o le 0.05. Faʻaaoga le tupuaga e tusi ai laʻu ata.
5.3.1 Aafiaga o le HPMC Seam Manline ma Faatoilaloina le Frozen le taimi i le paluga le mautinoa maualuga

Ata 5.1 Aafiaga ole HPMC Seam ma Badzen Teuina i le maualuga o le paluga le mautinoa
As shown in Figure 5.1, when frozen for 0 days, with the increase in the amount of HPMC added, the proofing height of the dough increased from 4.234-0.11 cm to 4.274 cm without adding HPMC. -0.12 cm (0.5% HPMC added), 4.314-0.19 cm (1% HPMC added), and 4.594-0.17 cm (2% HPMC added) This may be mainly due to HPMC Addition changes the properties of the dough network structure (see Chapter 2). Ae ui i lea, ina ua uma ona faʻaʻaisa mo le 60 aso, o le faʻamaoniga maualuga o le paluga faʻaititia i le eseese o tikeri. Specifically, the proofing height of the dough without HPMC was reduced from 4.234-0.11 cm (freezing for 0 days) to 3 .18+0.15 cm (frozen storage for 60 days); O le paluga na faʻaopopoina ma le 0.5% HPMC na faʻaititia mai le 4.27 + 0.12 Cm (froozen teuina mo 0 aso) i le 3.421 60 aso); O le paluga na faʻaopopoina ma le 1% HPMC na faʻaititia mai le 4.314-0.1 Fron (Faatosinaga CM (Canzens Inm (froball cham mo 60 aso); while the dough added with 2% HPMC woke up. The hair height was reduced from 4.594-0.17 cm (frozen storage for 0 days) to 4.09-±0.16 cm (frozen storage for 60 days). It can be seen that with the increase of the addition amount of HPMC, the degree of decrease in the proofing height of the dough gradually decreases. This shows that under the condition of frozen storage, HPMC can not only maintain the relative stability of the dough network structure, but also better protect the survival rate of yeast and its fermentation gas production activity, thereby reducing the quality deterioration of fermented noodles.
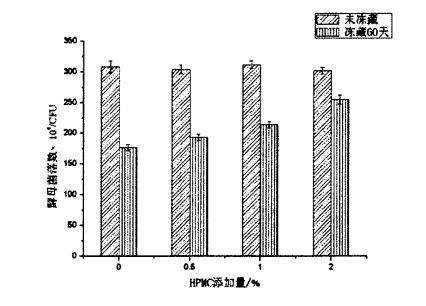
It can be seen from Figure 5.2 that there is no significant difference in the number of yeast colonies in samples with different contents of HPMC added without freezing treatment. E talitutusa lenei ma lenei taunuuga e fuafua e Heitmann, Zannini, & Went (2015) [180] [180]. However, after 60 days of freezing, the number of yeast colonies decreased significantly, from 3.08x106 CFU to 1.76x106 CFU (without adding HPMC); mai le 3.04x106 CFU i le 193x106 CFU (Faaopopo 0.5% HPMC); reduced from 3.12x106 CFU to 2.14x106 CFU (added 1% HPMC); reduced from 3.02x106 CFU to 2.55x106 CFU (added 2% HPMC). By comparison, it can be found that the freezing storage environment stress led to the decrease of the yeast colony number, but with the increase of HPMC addition, the degree of the decrease of the colony number decreased in turn. This indicates that HPMC can better protect yeast under freezing conditions. The mechanism of protection may be the same as that of glycerol, a commonly used strain antifreeze, mainly by inhibiting the formation and growth of ice crystals and reducing the stress of low temperature environment to yeast. Figure 5.3 is the photomicrograph taken from the 3M yeast rapid counting test piece after preparation and microscopic examination, which is in line with the external morphology of yeast.
Glutathione is a tripeptide compound composed of glutamic acid, cysteine and glycine, and has two types: reduced and oxidized. When the yeast cell structure is destroyed and died, the permeability of the cells increases, and the intracellular glutathione is released to the outside of the cell, and it is reductive. It is particularly worth noting that reduced glutathione will reduce the disulfide bonds (-SS-) formed by the cross-linking of gluten proteins, breaking them to form free sulfhydryl groups (.SH), which in turn affects the dough network structure. stability and integrity, and ultimately lead to the deterioration of the quality of fermented flour products. Usually, under environmental stress (such as low temperature, high temperature, high osmotic pressure, etc.), yeast will reduce its own metabolic activity and increase its stress resistance, or produce spores at the same time. A fetaui tulaga le siosiomaga mo lona tuputupu aʻe ma le toe gaosia, ona toe faʻafoʻi lea o le metablilism ma le alualu i luma. However, some yeasts with poor stress resistance or strong metabolic activity will still die if they are kept in a frozen storage environment for a long time.
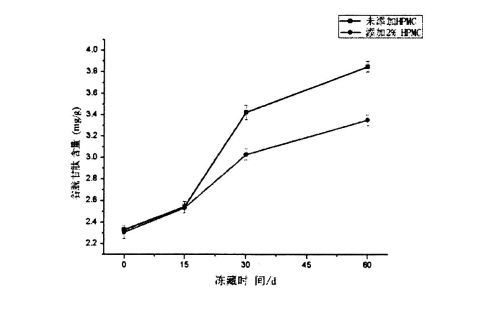
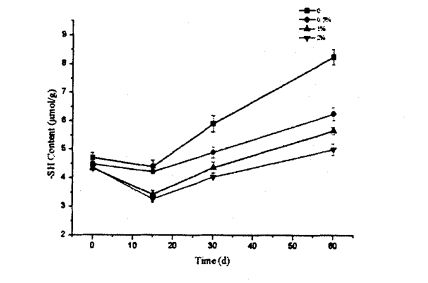
As shown in Figure 5.4, the glutathione content increased regardless of whether HPMC was added or not, and there was no significant difference between the different addition amounts. E ono mafua ona o nisi o le malosi o le mea leaga na 'ai masani ona fai le paluga e le lelei mafatiaga popole ma le faapalepale. Under the condition of low temperature freezing, the cells die, and then glutathione is released, which is only related to the characteristics of the yeast itself. It is related to the external environment, but has nothing to do with the amount of HPMC added. Therefore, the content of glutathione increased within 15 days of freezing and there was no significant difference between the two. However, with the further extension of the freezing time, the increase of glutathione content decreased with the increase of HPMC addition, and the glutathione content of the bacterial solution without HPMC was increased from 2.329a: 0.040mg/ g (frozen storage for 0 days) increased to 3.8514-0.051 mg/g (frozen storage for 60 days); while the yeast liquid added 2% HPMC, its glutathione content increased from 2.307+0 .058 mg/g (frozen storage for 0 days) rose to 3.351+0.051 mg/g (frozen storage for 60 days). This further indicated that HPMC could better protect yeast cells and reduce the death of yeast, thereby reducing the content of glutathione released to the outside of the cell. This is mainly because HPMC can reduce the number of ice crystals, thereby effectively reducing the stress of ice crystals to yeast and inhibiting the increase of extracellular release of glutathione.
5.4 Mataupu Mataupu
Taimi o le taimi: Oketopa-08-2022